Basic Structure of Atoms
2. Short Answer Test – Click Here
3. Kahoot! based on Video – Click Here

All atoms are composed of 3 main components – Protons, Electrons, and Neutrons. Protons are positively charged – Electrons, Negatively Charged and Neutrons – have no Charge.
The protons and neutrons are found in the core of an atom – its “NUCLEUS” while the Electrons are found around the nucleus in what are called – “electron orbitals”. While the protons and neutrons in the nucleus of the atom tend to be stable and unchanging the electrons in the orbitals are more fluid in there ability to exchange and share with other atoms.
Atoms are listed on the periodic table from 1 to 118.
The first 20 atoms of the periodic make up 99% of the atoms in all living things so we will focus on these first 20. An atoms number on the periodic table tells you how many protons are in the atom. So atom #1, “Hydrogen” has one proton, atom #2, Helium – has 2 protons, #3 Lithium has 3 protons, and so on. We already learned that protons are positively charged and found in the nucleus so that means Lithium (atom #3) has 3 positively charged protons in its nucleus. Now with all this positivity around we need to balance it out – and that’s where the electrons come in. Each electron in an atom adds a negative charge.

Lithium needs 3 electrons to balance the 3 protons in its nucleus and make it neutral
However, atoms aren’t always neutral. Atoms can sometimes have uneven numbers of protons and electrons – when this happens – they become charged. These charged atoms are called ions. If an atom has more protons than electrons it becomes a positively charged ion – also called a CATION. If it has more electrons than protons it becomes negatively charged and is called an ANION.
Basic Structure of the Lithium Atom

Lithium needs 3 electrons to neutralize its 3 protons. With only 2 electrons it becomes Li+ a Lithium cation.
So why would an atom become an ion rather than remaining in its neutral form? Well, the answer to that is – STABILITY. All atoms must attain a stable electron configuration. And in order to understand what this means we need to look at electron orbitals and learn a few rules.
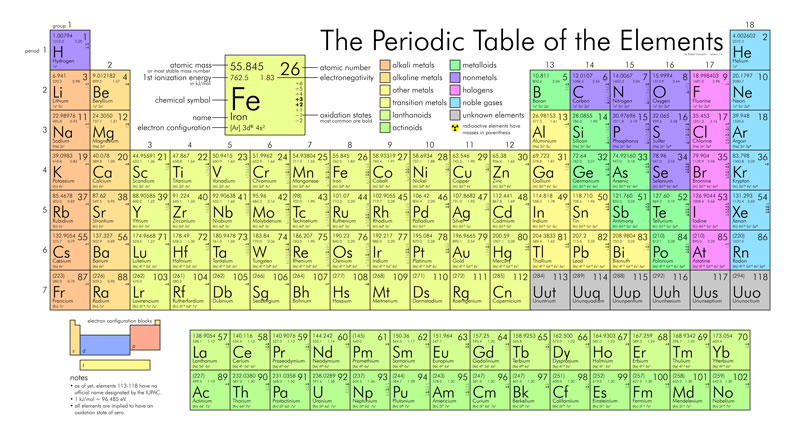
I mentioned before that electrons are found around the nucleus in orbitals. But these orbitals have some very strict rules about how many electrons they can hold. The first orbital holds a maximum of 2 electrons and is stable only if it is completely filled with two electrons. The 2nd orbital can hold a maximum of 8 electrons and also is only stable if all 8 are filled. So partially filling this orbital with 3 electrons or 5 electrons is unstable – it needs a complete 8 electrons. The third orbital, like the second is also stable when it holds 8 electrons – any fewer, just won’t cut it. So for the first 20 atoms or so, on the periodic table – the only stable electron configurations are:
- no electrons
- 2 electrons in the first orbital
- 2 electrons in the first orbital and 8 in the second orbital – a total of 10 electrons
- 2 electrons in the first orbital, 8 in the second orbital, 8 in the third orbital – a total of 18 electrons.
All of the first 20 atoms have to find some way to attain one of these 4 basic stable electron configurations. Another way of saying that is all of these first 20 atoms must find some way to be surrounded by 0, 2, 10, or 18 electrons. So lets go back to the question why would an atom become an ion rather than staying in its neutral form? The answer – To attain a stable electron configuration – or in other words to attain a total of 0, 2, 10, or 18 electrons. Let’s us some examples to demonstrate what this means.
Basic Structure of Atoms – Neon – Atom #10

Neon, atom #10 – has 10 protons in its nucleus. To balance the positive charges it needs 10 electrons – 2 in the first orbital and 8 in the second. This is one of the 4 stable electron configurations I just mentioned – So Neon is STABLE by itself as a neutral atom.
That also applies to Helium #2 on the periodic – which is completely stable in its neutral form and Argon #18 – also stable when it has an equal number of protons and electrons. Those are the only 3 atoms in the first 20 on the periodic table that are stable in their neutral form. Because of this stability these atoms are very non-reactive and are call the inert gases. They are found in the last column of the periodic table – the column that represents neutral atoms with stable electron configurations.
Basic Structure of Atoms – The noble gases

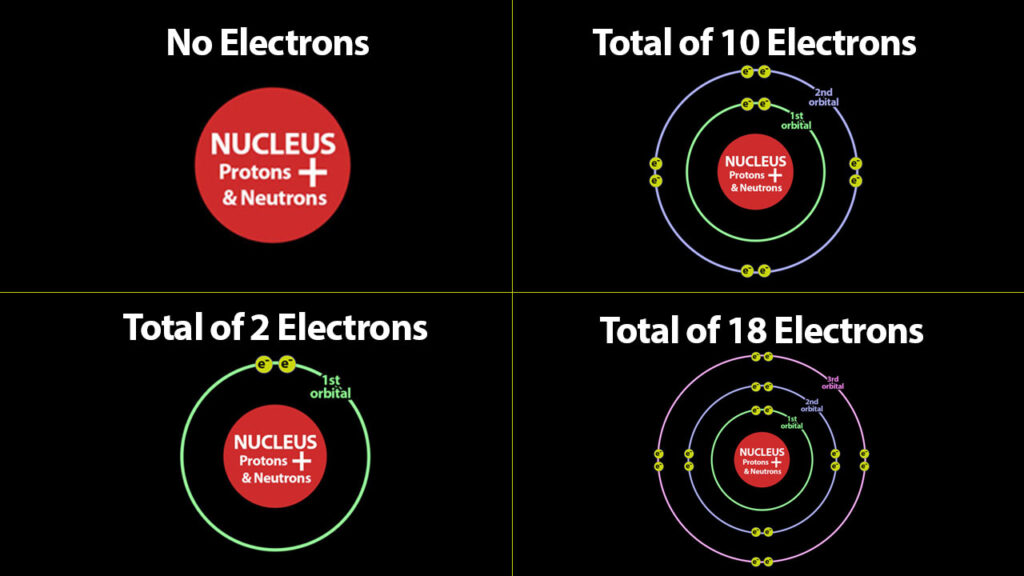
Basic Structure of Atoms – Sodium – Atom #11
Now lets move on to some atoms that aren’t as stable. Sodium, – #11 – so how many protons does it have? – that’s right 11! How many electrons does it need to balance these protons and make it neutral? 11. However, with 11 electrons, is the atom stable?
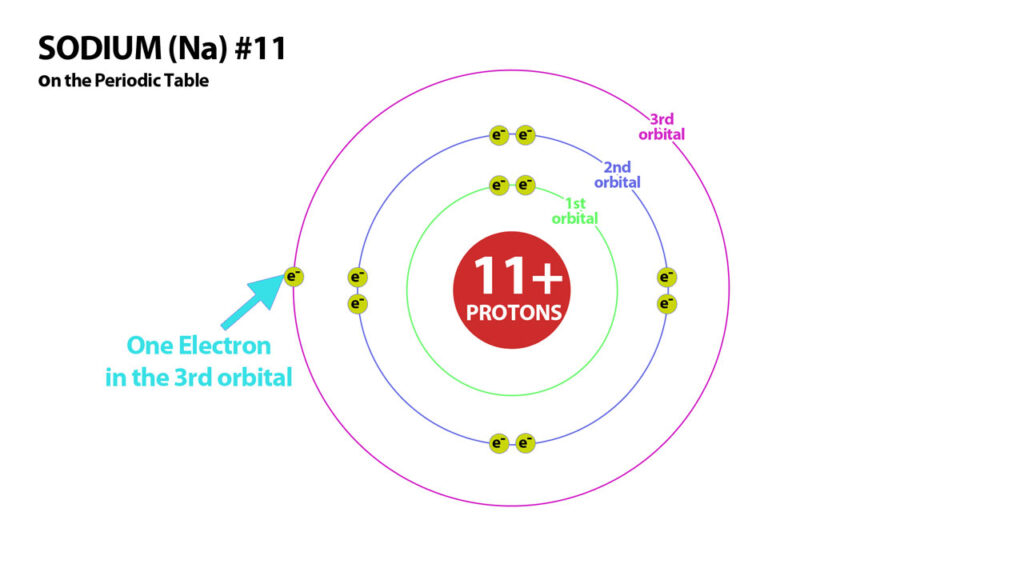
Neutral Sodium Atom – with 11 electrons and 11 protons, sodium is neutral but not stable.
The above atom is not stable. It is neutral with 11 electrons, but it is not stable because it has one electron in its outer orbital. However if we remove this one electron, it now has a stable configuration or a total of 10 electrons – 2 in the first orbital / 8 in the second. But it’s no longer neutral because it now has 11 protons and only 10 electrons.

Neutral Ion – with 10 electrons and 11 protons, sodium form a stable 1+ sodium ion.
This sodium ion is incidentally – the most abundant cation in the body. Similar to Sodium are Hydrogen, Lithium, and Potassium. Hyrogen #1 – has one electron in its neutral form – if it loses this electron it attains a stable configuration of 0 electrons and becomes a 1+cation. Lithium #3 has 3 electrons when neutral and can lose one of its electrons to attain a stable configuration of 2 electrons. And Potassium #19 – has 19 electrons when neutral and so can lose 1 to attain the stable configuration of 18 Electrons. These first column atoms are all in the same situation of having one electron too many for a stable configuration. They all therefore can lose one electron to attain stability thereby becoming 1+ ions.
Basic Structure of Atoms – First Column Atoms – 1+ ions

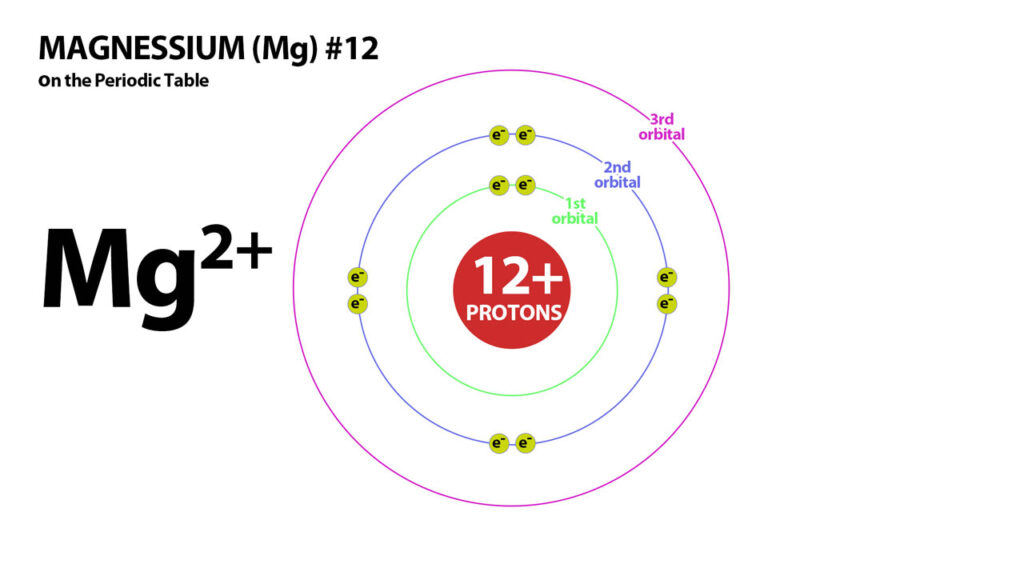
Basic Structure of Atoms – Magnessium – Atom #12
Magnessium – #12 has 12 protons in its nucleus. It needs 12 electrons to balance its charges and become neutral.
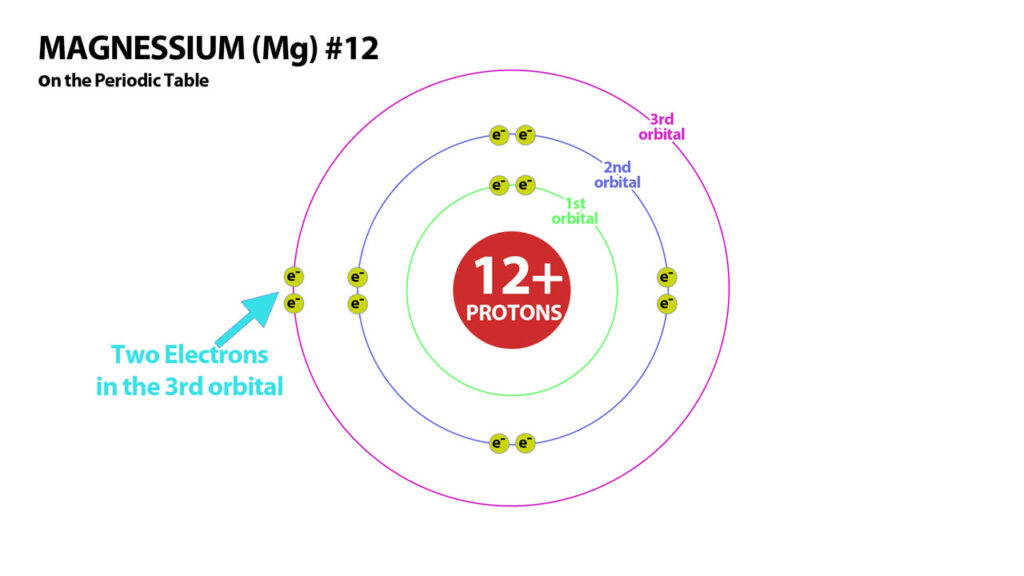
Magnessium has 2 electrons in the outer shell in its neutral form.
But 12 electrons is not stable so Magnessium needs to lose 2 electrons to attain a stable electron configuration. Magnessium, therefore becomes a 2+ ion because it has 2 more protons than electrons.Similarly Calcium (#20) has 20 protons and so with 20 electrons is neutral but not stable. It, like magnessium also loses its 2 electrons to become a 2+ Cation.
Basic Structure of Atoms – second Column atoms – 2+ ions

These second column atoms all need to lose 2 electrons to attain a stable configuration and so all become 2+ ions.
Flourine is #9 on the periodic table – It needs one more electon in its 2nd orbital to attain 10 electrons – a stable number of electrons.
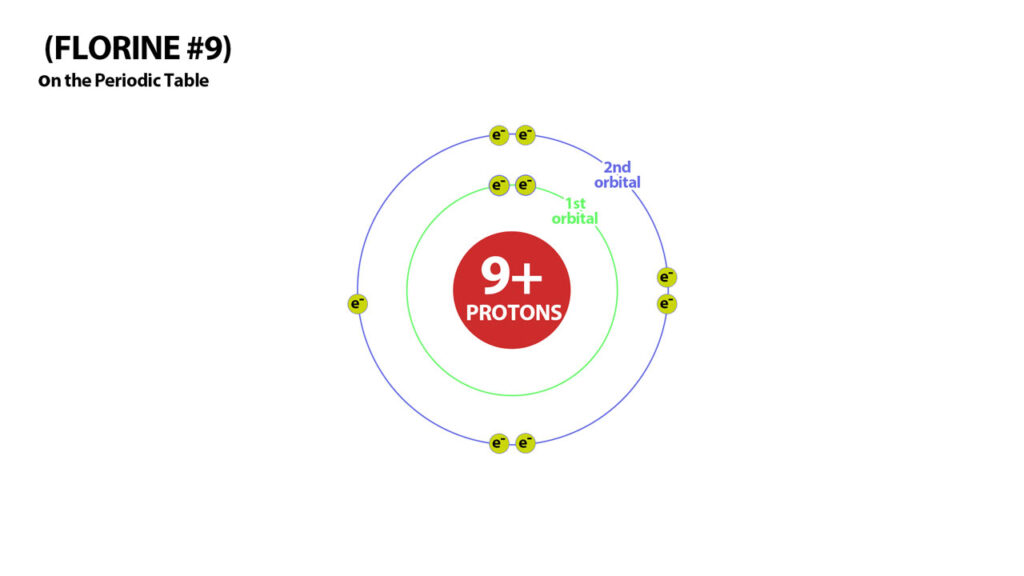
Neutral Flourine has 9 electrons and 9 protons – it needs one more electron for stability
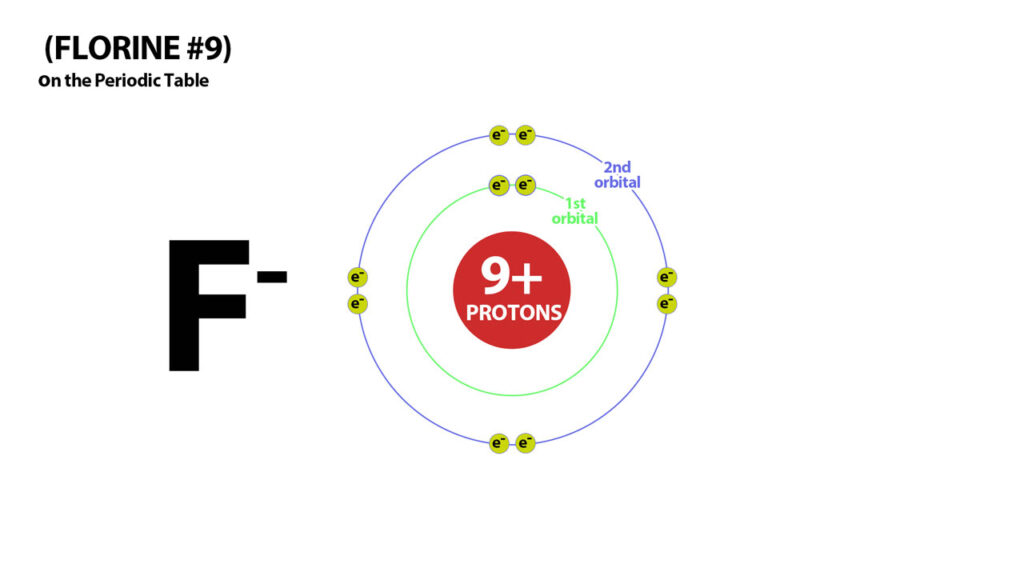
By adding an extra electron Florine attains stability but becomes a negatively charged Flouride ion – an anion.
Similarly Chlorine (#17) needs 17 electrons to be neutral – with one more electron it attains a stable electron configuration of 18 electrons but becomes negatively charged – a 1- anion. These atoms in the 2nd last row therefore all become 1- ions because they all need one more electron to attain a stable number.
Basic Structure of Atoms – Carbon – Atom # 6
Lets now take a look at the central atom in Organic Chemistry – Carbon – to see what it does to attain a stable number of electrons. Carbon (#6) on the periodic table needs 6 electrons to balance its 6 protons. So it has 2 electrons in the first orbital and 4 electrons in the second orbital. To become an ion with a stable electron configuration it must either lose 4 electrons and become a 4+ ion or gain 4 electrons to become a 4- ion. That much charge in a small atom unfortunately also creates an unstable situation. So by stabilizing the electron configuration it would create an unstable charge.

The Basic Structure of the Carbon Atom – the central atom of organic chemistry.
So WHAT DOES CARBON DO TO STABILIZE ITSELF. Another way for atoms to attain a stable electron configuration is Bonding. Bonds are a way for an atom to share electrons with other atoms so they can both compensate for a insufficient number of electrons in their outer shell.
To better understand the details of how bonds allow atoms to share electrons and stabilize their outer shells –
click here to watch our next lecture on bonding.
2. Short Answer Test – Click Here
3. Kahoot! based on Video – Click Here
