Covalent Bonding
2. Short Answer Test – Click Here
3. Kahoot! based on Video – Click Here
This Lecture expands upon the information presented in the previous video / lecture “Atomic Structure Clearly Explained – CLICK HERE TO WATCH“. If you have not yet watched that video it may be helpful to review that video first before moving onto this material.

In this lecture we will be discussing covalent bonding by looking at the bonds created by the 4 main atoms that make up 96% of all living things – Carbon, Hydrogen, Oxygen and Nitrogen.
Covalent bonding of Carbon

Carbon, atom #6 on the periodic table is the central atom of Organic Chemistry. It has 6 protons and 6 electrons – 2 electrons in its first orbital and 4 in its second orbital.
Carbon needs 4 more electrons to attain a stable electron configuration. One way for it to attain these 4 extra electrons is by making bonds. Covalent Bonding is a way for an atom to share electrons with other atoms so they can both compensate for an insufficient number of electrons. For each electron that is missing, an atom needs to make one bond. So to compensate for its 4 missing electrons carbon needs to make 4 bonds or – in other words it needs to share 4 electrons with other atoms.

Carbon is missing 4 electrons in its outer shell so always needs to share 4 electrons – make 4 bonds. THIS IS WHY CARBON ALWAYS MAKES 4 COVALENT BONDS
Covalent bonding of Hydrogen

Hydrogen has one proton in its nucleus and 1 electron in its first orbital.

It needs one more electron to attain a stable configuration so Hydrogen always makes 1 bond.
Covalent bonding of Oxygen

Oxygen with 8 protons in its nucleus and 8 electrons needs 2 more electrons for stability. Oxygen therefore always make 2 bonds.
Covalent bonding of Nitrogen

Nitrogen #7 on the periodic table has 7 protons and 7 electrons in its neutral form. So Nitrogen can attain stability by making 3 bonds.
So lets use this information to build some common molecules seen in biology and nature. Lets start with Hydrogen gas.

Two hydrogen atoms can bind to one another to form a stable gas (Hydrogen gas) H2. Each atom is one electron short of having a stable number of electrons so by sharing their electrons they both attain a stable number of 2 electrons.
Another very simple molecule is water. Oxygen needs to make 2 covalent bonds – it can do this by binding to two hydrogen atoms that both need to make 1 bond. After bonding with 2 hydrogens, oxygen attains a stable number of 8 electrons in its 2nd orbital and each hydrogen attains stability with 2 electrons. When an Oxygen atom binds to 2 hydrogen atoms, it forms H20, Water.

The basic structure of water showing the 2 covalent bonds formed between the oxygen and hydrogens. Each covalent bond represents the sharing of electrons between 2 atoms.

Nitrogen needs to make 3 covalent bonds to attain a stable electron configuration so can join with 3 Hydrogen atoms to form NH3, Ammonia.

Carbon, likes to make 4 covalent bonds so can bind with 4 hydrogens to create CH4 – methane.
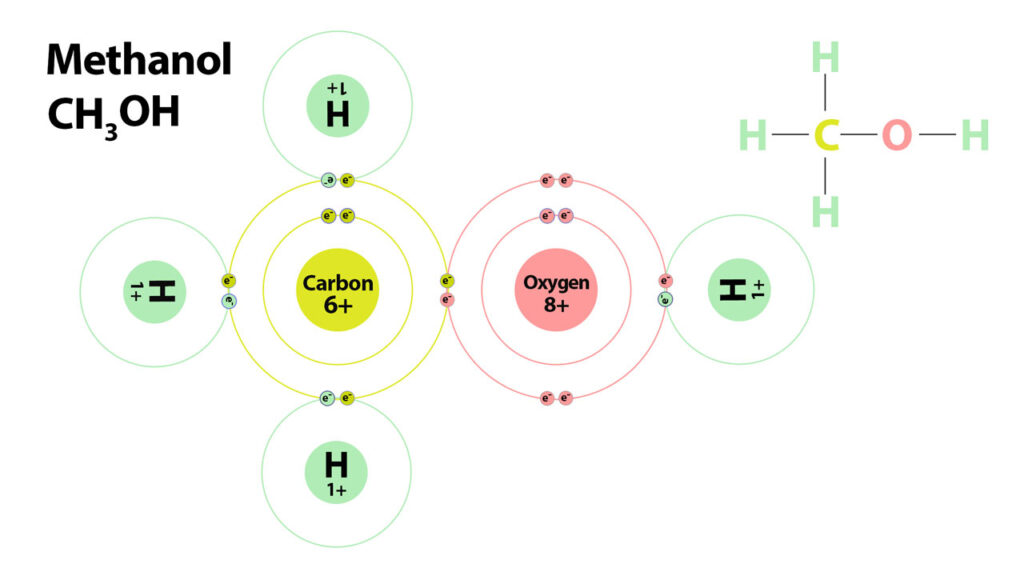
Alcohols are created by adding an OH group (oxygen and hydrogen) to carbon molecules. For example if you add an OH group to methane you get Methanol.

Atoms can sometime partake in double bonds by sharing more than one electron with the same atom. For example – Oxygen, which needs to make 2 bonds for its stability can make two bonds with another oxygen atom to create gaseous oxygen (O2). This is the 2nd most abundant gas in our atmosphere making up about 21% of gases in the air.

Triple bonds are also possible between atoms that are able to make 3 or more bonds like carbon or nitrogen. For example, Nitrogen which need 3 bonds for its stability can make 3 bonds with another Nitrogen atom to create Nitrogen Gas (N2). Nitrogen gas is the most abundant gas in the atmosphere accounting for 78% of the air we breath.
Covalent Bonding in Larger Organic Molecules
Now we will move on and take a look at some larger molecules found commonly in the body.

Fatty acids are large molecules found in most plants and animals that are composed of carbon, hydrogen and oxygen. They are composed of a chain of carbons surrounded by hydrogens and at one end they have oxygens bound to the carbon.
Fatty acids are the most abundant fats in our body’s and all have this same basic structure of a string of carbons surrounded by hydrogen with what is called a carboxylic acid group at one end. Notice that all the carbons in this molecule have 4 bonds around them – all the hydrogens one bond and all oxygens have 2 bonds.
Lastly we will look at the structure of amino acids. Amino acids are the building blocks of proteins and are some of the most important and common molecules in the body. We will create the amino acid Glycine. Glycine is composed of a central carbon atom that is surrounded by 2 hydrogens and a carboxylic acid (COOH) and an amino group (NH3).

Glycine is composed of a central carbon atom that is surrounded by 2 hydrogens, a carboxylic acid (COOH) group and an amino group (NH3).
Notice that in these larger molecules, just like in the smaller molecules, the same rules apply. Carbons all make 4 covalent bonds, Nitrogen atoms make 3 covalent bonds, oxygens make 2 and hydrogens 1. Because these 4 atoms make up 96 % of the atoms in living things, understanding these basics allows us to understand the covalent bonding that occurs in virtually all the molecules in the body!
2. Short Answer Test – Click Here
3. Kahoot! based on Video – Click Here
