Fats / Lipids – Biochemicals in Living things
2. Short Answer Test – Click Here
3. Kahoot! based on Video – Click Here
There are 4 main classes of biochemical molecules in the body. Fats, Carbohydrates, Proteins and Nucleic acids. Here we will be discussing FATS otherwise known as LIPIDS.
Basic Structure of Fats and Lipids
Fats are almost exclusively made from just 2 of the atoms on the periodic table – Carbon and Hydrogen
Because carbon and hydrogen always form non-polar bonds with one another this also means fats are non-polar molecules and that they do not mix well with other molecules in the body which are mainly polar.

Fats are non-polar molecules that do not mix well with other molecules in the body.
THE MAIN FATS IN THE BODY
The main fats in the body include:

Fatty acids are long chains of carbon surrounded by hydrogens. At the end of all fatty acids is a carboxylic acid group. There are between 4 and 24 carbons in the fatty acids commonly found in the body.
Saturated and Unsaturated Fatty Acids

If a fatty acid has no double bonds between its carbons it is called a saturated fatty acid.

Fatty acids with double bonds between their carbons are “Unsaturated Fatty Acids”
Fatty acids with only one double bond are called monounsaturated fatty acids.

The omega-9 monounsaturated fatty acid “Oleic Acid” makes up about 70% of the fatty acids found in olive oil. It is called an omega-9 fatty acid because its first (and only) double bond is at the 9th carbon from the omega end.

Fatty acids with multiple double bonds are called polyunsaturated fatty acids. This is the polyunsaturated fatty acid alpha linolenic acid. This is an omega 3 fatty acid because its first double bond is at the 3rd carbon from the omega end.
Also notice that these double bonds cause the fatty acid to have a bent shape. This is important because the bent shape of unsaturated fatty acids means they do not pack together as well as saturated fats which have a straight shape. For this reasons unsaturated fatty acids do not form solids as easily and so tend to be liquids at room temperature.
Highly saturated fats like butter and coconut oil are solid at room temperature. Whereas fats that contain large amounts of polyunsaturated fatty acids – like flax oil – will remain a liquid even when put in the freezer. Also notice when you add a double bond two hydrogens are removed from the molecule. Unsaturated fatty acids therefore also have fewer hydrogens.
Another important characteristic of unsaturated fats is that they are more unstable than saturated fats. Saturated fats are very stable – they don’t tend to react with other molecules, even when heated or exposed to light. The double bond in unsaturated fatty acids however, makes unsaturated fats more reactive especially when heated. For this reason highly unsaturated fats like flax oil spoil easily and should be stored in the fridge or freezer and in a dark container.
Trans Fatty Acids
Unsaturated fatty acids can also sometimes form what are called TRANS FATS. Trans fats are unsaturated fatty acids that have changed shape at their double bond.

Unsaturated fats usually have a bent shape at their double bond. This is called the cis shape. The cis shape puts the two hydrogens on the same side of the double bond.
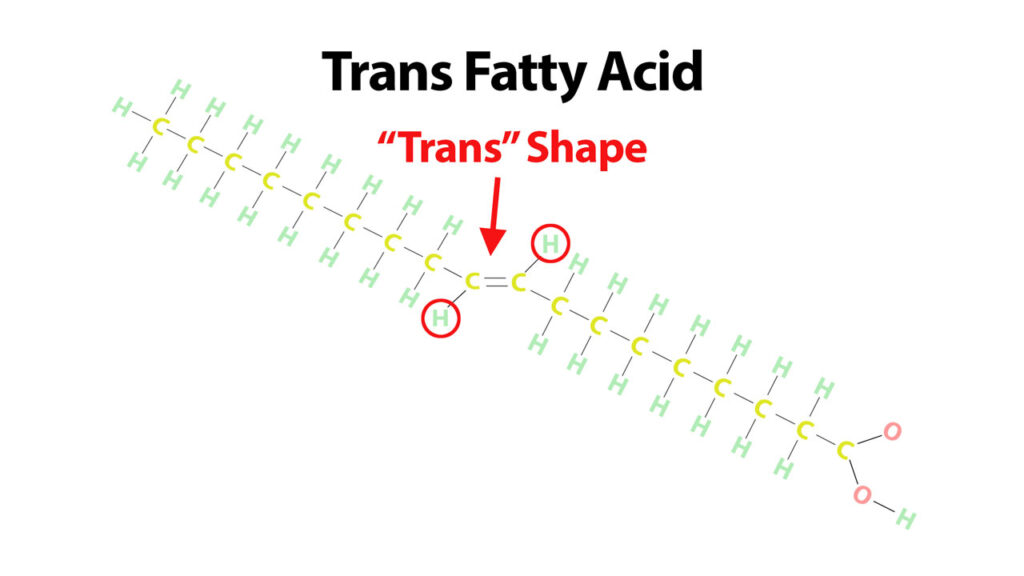
However, if we twist this unsaturated fatty acid at the double bond the two hydrogens end up on opposite sides of the double bond. We call this the trans shape and we call the fatty acids that have double bonds in this configuration, “trans fatty acids”.
These fats are concerning because these straightened fatty acids are not commonly built by most living things and so are not found in large quantities and natural foods. Trans fats are formed in large quantities and partially hydrogenated fats. Research started linking high trans fat consumption with increased risk of cardiovascular disease and so to reduce their presence in foods partially hydrogenated oils have been banned in many countries including Canada and the United States.
Essential Fatty Acids
There are also certain unsaturated fatty acids that are essential to our health called essential fatty acids. These include the omega-3 fatty acids and omega-6 fatty acids. Our body has the ability to make many fatty acids from smaller building blocks but cannot make omega-3 or omega-6 fatty acids. We must obtain these from our diet. The omega-3 and omega-6 fatty acids are polyunsaturated fatty acids.

Omega-3 fatty acids have their first double bond at the third carbon from the omega end.

Omega-6 fatty acids have their first double bond at carbon 6.
Omega 3 and omega 6 fatty acids have vital functions in our body and assist with our immune health, brain health, and much more.

Omega-6 fatty acids have their first double bond at carbon 6.
FATS IN THE BODY: 2) triglycerides
In the body fatty acids are generally not found by themselves but rather are found as a part of two other very important molecules Triglycerides and Phospholipids.
Lets take a look at TRIGLYCERIDES. When our body has extra fatty acids we store them by creating molecules called triglycerides.

Triglycerides are created by taking a molecule called glycerol and linking 3 fatty acids to it.
Triglycerides are the most abundant fats in the body – they are used mainly as a way to store extra energy and are stored in cells called adipocytes found around organs and under our skin.

The second place we find fatty acids is a part of Phospholipids. Phospholipids have a very similar structure to triglycerides, however, instead of having 3 fatty acids linked to the glycerol molecule, in phospholipids one of the fatty acids is replaced by a phosphate molecule.
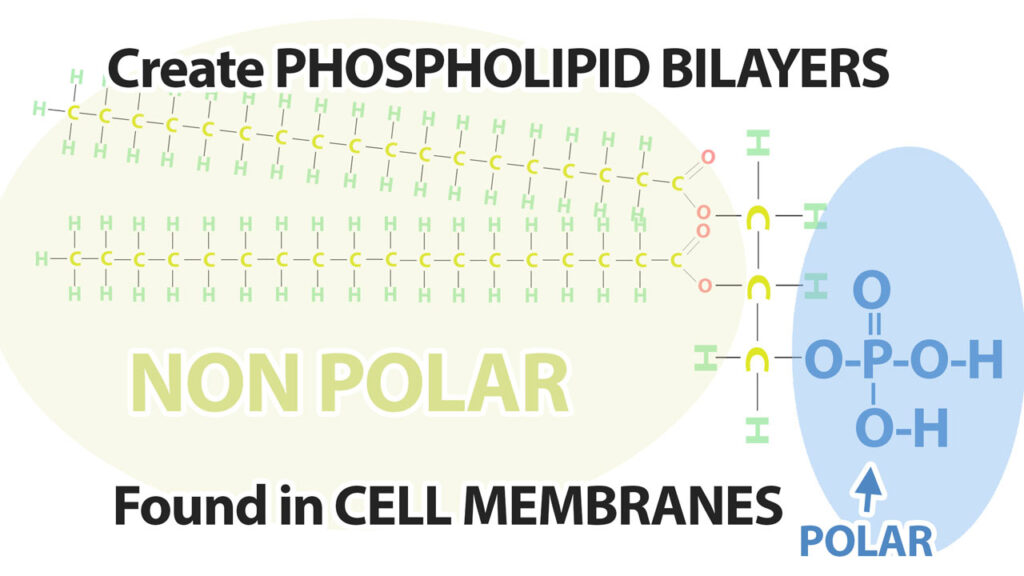
The phosphate portion of the phospholipid is polar and so is capable in mixing with water, while the fatty acids are non-polar and so are hydrophobic – do not mix will with water. These phospholipids create the phospholipid bilayer found in all cell membranes.
Another very important fat in the body is Cholesterol. Cholesterol has many very important functions in the body. While some cholesterol in the body comes from our diet, we actual produce approximately 80% of our cholesterol.

The carbons in hydrogen loop around to create 4 rings. Like other fats cholesterol is composed almost exclusively of carbon and hydrogen with only one oxygen in the entire molecule.
Cholesterol itself has important functions in cell membrane structure and it is also found in bile and helps to emulsify fats. Cholesterol is the precursor for the production of the body’s steroid hormones which include – estrogen, aldosterone, testosterone and cortisol.

Basic structure of the steroid hormones – estrogen, aldosterone, testosterone and cortisol.
The final fat molecules we will discuss are the eicosanoids. These molecules are produced from fatty acids stored in the cell membrane of cells.

Eicosanoids are a powerful class of molecules that are created when we modify certain fatty acids in the body – namely arachadonic acid and EPA. These fatty acids can be broken down and converted into a plethora of tiny molecules that mediate many cellular processes such as inflammation, fever, and pain to name a few.
