The Importance of Polarity In The body
2. Short Answer Test – Click Here
3. Kahoot! based on Video – Click Here
Why is Polarity Important? #1 Polarity is essential to cell survival
Polarity is one of the most important characteristics that determines the behavior and function of molecules in the body. One of the main functions of NON-POLAR molecules is their role in membrane permeability. Cell membranes are composed of a layer of non-polar molecules that surround the outside of the cell. Because polar molecules do not mix will with non-polar molecules this layers of fats around the outside of the cell creates a barrier for the entrance of polar molecules. Thus polar substances can not pass through this fatty membrane – the fat creates an impermeable barrier to polar substances. Similarly charged substances like sodium ions and chloride ions also can not cross this fatty barrier.
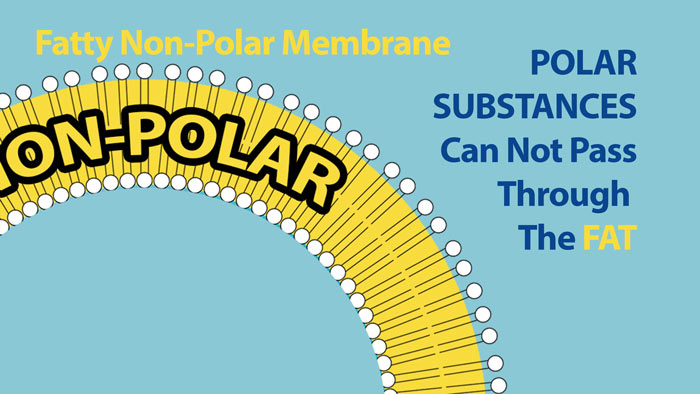
Polar substances can not pass through the fatty Non-Polar core of the cell membrane.
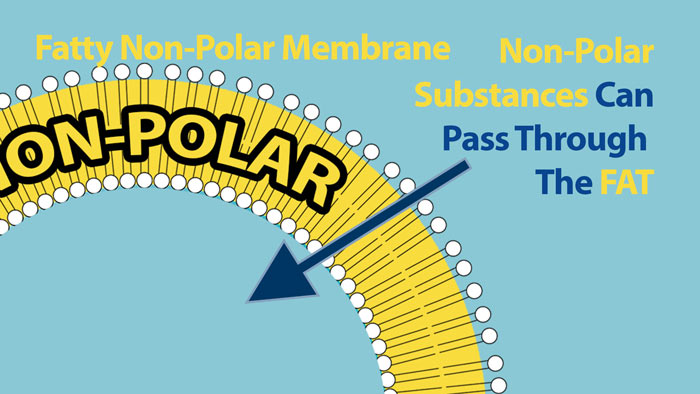
Non-Polar substances however, can pass through the Non-Polar core of the cell membrane.
Polar substances can enter cells, but they need help. They need a passageway to assist with their entrance. For this reason cell membranes have special carrier proteins and channels that act like doors that can open and close to allow passage of very specific polar molecules into and out of the cell. In this way the cell has a large degree of control over what enters and what leaves. Cells exist in a watery environment so virtually all the molecules around the cell are polar so this fatty membrane creates an effective barrier to most molecules trying to enter the cell.
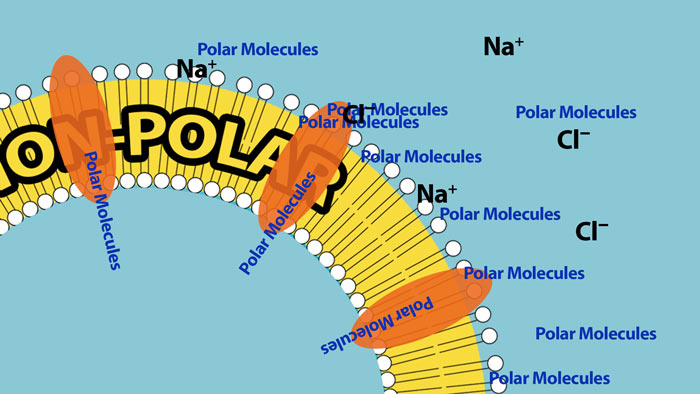
Although Polar substances can not penetrate the fatty non-polar portion of the membrane they can still pass into the cell through special channels/carriers that allow passage of very specific polar substances.
Why is polarity important? #2 To help control the entrance of substances into the body.
Epithelial tissues are composed of layers of cells and line surfaces in the body. Epithelial tissues often serve as a barrier in order to control the passage of substances into and out of the body and into and out of different compartments within the body. For example, your epidermis is the epithelial tissue found on the surface of your skin. It is composed of many layers of cells. These cells form a very effective barrier against polar substances.
Most substances outside our bodies are polar – and so this barrier to polar substances forms a protective covering that resists the entrance of most substances. However, non-polar substances (fatty substances) can penetrate through the skin and enter the body. Fat soluble vitamins, fat soluble chemicals and agents absorb through the cells and into the body. Medications can be applied topically if they are non-polar because they can be absorbed through the skin – nicotene patches and estrodiol patches are examples of non-polar meds that can be administered through the skin.

The Skin is made of Many cells and so resists the passage of polar substances but non-polar substances can penetrate the skin.
Why is Polarity Important? #3 it influences the behavior of molecules inside the body.
Non-polar molecules in the body also need to be treated differently because they will not mix well with the watery polar environment. For example, fats in the body like triglycerides and cholesterol are wrapped up into structures called lipoproteins in order to be carried in the blood. The lipoproteins hold the fat molecules in their core so they can be carried through the blood without clumping together. HDL, LDL and VLDL are examples of fat carrying lipoproteins found in the blood.
Oxygen and carbon dioxide are also non-polar and so don’t mix well with the body’s water medium. In order to hold the massive amounts of Oxygen required to sustain life we rely on a protein called hemoglobin to hold the oxygen and carry it through the body. Similarly Carbon Dioxide is non-polar and so also does not dissolve in blood. To carry large amounts of Carbon Dioxide it is converted into bicarbonate ions which then can dissolve in the blood.
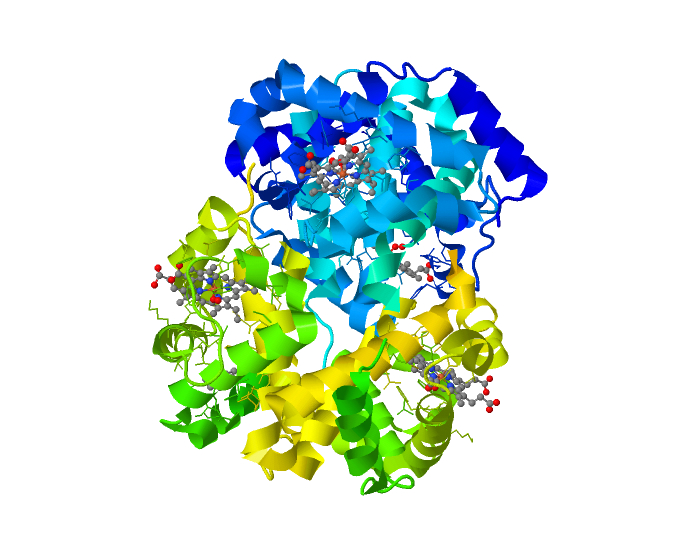
Oxygen is a non-polar molecule and so does not dissolve well in the watery medium of the body. Oxygen is therefore carried in the blood on a protein called Hemoglobin.
These are just a few reasons why polarity is such an important property in the body and how understanding this property helps us to better appreciate the behavior of molecules in the body.
